Examples of Open System in Thermodynamics | Open System Examples

In thermodynamics, an open system is a system that continuously interacts with its environment. Open systems are found all around us in our everyday lives. From the steam engine to the clothes dryer, there are many examples of open system in thermodynamics.
In this blog post, we will explore 10 everyday open system examples in thermodynamics. We will look at how these open system examples work, how they interact with their environment, and why they are important. With these examples, you will better understand how thermodynamics works and its application in our day-to-day lives.
10 Daily Life Examples of Open System in Thermodynamics
Examples
Here are the 10 daily life open system examples in thermodynamics.
- Air Conditioner
- Automobiles
- Refrigerator
- Heat Engines
- Internal Combust Engines
- Gas Turbines
- Electricity
- Jet Engines
- Solar Energy Collectors
- Geothermal Power Plants
-
Air Conditioners

Air conditioners are an example of open systems in thermodynamics. In an open system, energy can flow in and out of the system, while the matter is not necessarily conserved. This makes air conditioners particularly efficient at cooling. An air conditioning system consists of an evaporator that absorbs heat from the air and a condenser that releases it outside.
The compressor pumps refrigerant through the two components, allowing for an efficient exchange of thermal energy. Heat is extracted from the interior space by the evaporator, cooled, and then released to the exterior via the condenser. This exchange of thermal energy between the two sides of the system makes air conditioners an example of an open system in thermodynamics.
- Automobiles

In the case of automobiles, energy is exchanged as heat, and matter is exchanged as exhaust gases. The engine takes in fuel, converts it to heat, and then uses that heat to power the car. Exhaust gases are then released back into the atmosphere, making it an open system.
What is more, energy from the car’s motion is used to turn the wheels, allowing it to move forward. This is just another example of an open system in thermodynamics, where energy and matter are constantly exchanged.
- Refrigerators

Refrigerators are one of the most common examples of open systems in thermodynamics. Refrigerators are open systems because they move energy in and out of the interior. For them to cool food and drinks, heat must be removed from inside the fridge and transferred to the environment outside.
This is accomplished through a process called heat exchange, where hot air from inside the refrigerator is exchanged with cold air from the outside. This exchange is enabled by what is an open system in thermodynamics: a series of vents and fans that keep the exchange of energy consistent.
- Heat Engines
These systems use the flow of energy from a hot reservoir to a cold reservoir to generate work or power. Heat engines rely on the differences between these two temperatures to convert heat energy into mechanical energy.
In a typical heat engine, a cylinder filled with gas or liquid is heated by a hot source and then cooled by a cold source. The expansion and contraction of the material inside the cylinder drive a piston that can be used to turn a shaft, create motion, and generate power. Examples of heat engines include internal combustion engines and steam engines.
- Internal Combustion Engines
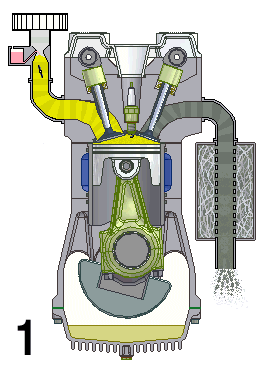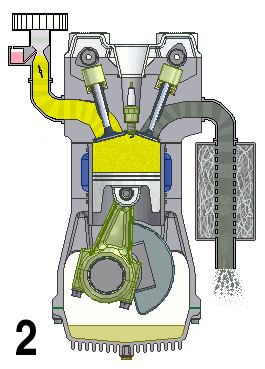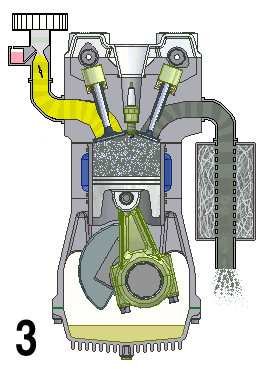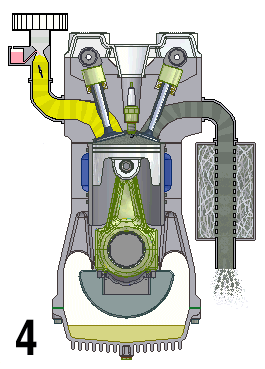
Internal combustion engines are a type of open system in thermodynamics and are examples of heat engines. This means that they are engines that take in a mix of air and fuel and combine them to produce energy. This energy is then used to drive the vehicle forward.
The engine works by drawing in air and fuel through an intake valve and compressing it in a cylinder, which is then ignited by spark plugs or diesel injectors. The burning of the fuel generates heat, which expands and creates pressure to turn the crankshaft and generate motion. The exhaust gases are then expelled out of the engine through an exhaust valve.
The key point about internal combustion engines as an open system is that energy is taken from the environment and converted into useful work.
- Gas Turbines
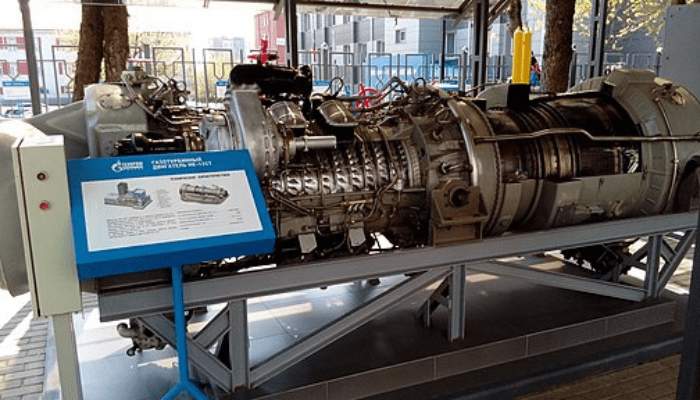
Wulfson, CC BY-SA 4.0 via Wikimedia CommonsIt is a type of combustion engine where hot gases move through a series of blades that spin a shaft, producing mechanical energy. The gases enter the system, undergo combustion, and then exit the system, creating a continuous cycle of energy production.
Gas turbines are used to power aircraft, generate electricity, and in certain industrial applications. Gas turbines are highly efficient, allowing for the conversion of more than 70% of the energy from the fuel into mechanical work.
The exhaust gases also contain valuable energy which can be used to power other processes in the same system. This makes gas turbines highly efficient and cost-effective, making them a great choice for many applications.
- Electricity

Electricity is another example of an open system in thermodynamics that uses energy to power everyday appliances and gadgets. This energy comes from sources such as coal, nuclear power, solar, and wind. As energy is used, it is converted into heat, light, sound, and motion, which can be used to perform work.
In an open system, energy is exchanged between the source of electricity and its environment, resulting in a change in temperature and pressure. This exchange allows the system to maintain a stable equilibrium, with energy being conserved over time.
- Jet Engines

Jet engines take in air from the atmosphere, heat it, and eject it out of the back of the engine. This process creates a thrust that propels the aircraft forward.
The air taken in by the jet engine is not just used for combustion; it is also used to cool the engine down as it passes through various stages in the engine. The hot air is then expelled out of the back of the engine.
This process creates a continuous flow of air, which is what makes a jet engine a perfect example of an open system in thermodynamics.
- Solar Energy Collectors

Solar energy collectors work by collecting the sun’s rays and transferring them into usable energy for homes and businesses. The heat generated from these collectors is used to either heat water or power other appliances.
These systems operate through a process of absorption, conversion, and emission. In the absorption phase, light from the sun is absorbed by photovoltaic cells.
The energy from the sun is then converted into electrical energy in the conversion stage. Finally, in the emission stage, this energy is released as heat. Solar energy collectors are a great way to harness the power of the sun and convert it into usable energy for various applications.
- Geothermal Power Plants

Geothermal power plants use heat energy from the Earth to generate electricity. They are an example of an open system in thermodynamics. In geothermal power plants, heat energy is extracted from the Earth’s surface and used to turn a turbine generator that creates electricity. The heated water and steam then travel back into the Earth, completing the cycle.
Geothermal power plants are considered an environmentally friendly form of energy production since they have zero emissions and no impact on the environment. Additionally, geothermal energy is a renewable energy source, meaning it will never run out.
Recommended Read:
Congratulations, you have read the complete article related to Examples of open System in thermodynamics. If you have any doubts or queries, feel free to comment below. We will respond as soon as possible.
Or Email Us At [email protected]
Any topic you want us to cover? Let us know.